Vietnamese volunteers get the second shot of Nano Covax vaccine in second trial phase
| Vietnam encourages eligible enterprises to import Covid-19 vaccines | |
| Delivery of first COVID-19 vaccine shipment from COVAX Facility delayed | |
| China approves inhaled CanSino vaccine for clinical trials |
Volunteers receive second shots of Nano Covax in second-stage of human trials
According to Vietnam News, the Military Medical University under the Ministry of National Defence on Thursday began giving the second shots of the Nano Covax COVID-19 vaccine in the second trial phase to 26 volunteers who received the first jabs between February 26 and March 10.
Nano Covax developed by the Nanogen Pharmaceutical Biotechnology Company is Vietnam’s first candidate vaccine to reach the human trial stage.
According to Associate Prof. Dr. Chu Van Men, Director of the Military Medical University’s Centre for Clinical Trials and Bioequivalence, after receiving the first shots, volunteers exhibited symptoms such as pain at the injection point, a light fever, muscle aches, joint pain, and fatigue, but did not require medical intervention.
The health of the 560 volunteers remains stable, he said, adding that Nano Covax is safe and the volunteers are ready for their second shots.
The second trial is being organised simultaneously at the Military Medical University and the Pasteur Institute in HCM City, with volunteers including those with mild background ailments such as hypertension, diabetes, and cardiovascular issues.
The Military Medical University will submit the preliminary test results to the Ministry of Health and the National Council for Ethics in Biomedical Research in May for review and evaluation, before conducting the third phase of trials.
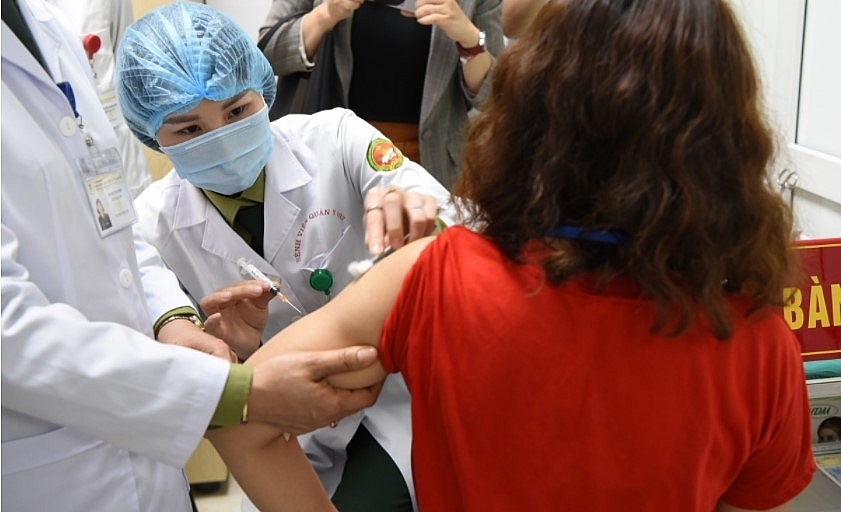 |
| A volunteer receive the second shot of the Nano Covax vaccine. Photo: VOV News. |
A homegrown COVID-19 candidate vaccine that is “relatively safe” for the volunteers
Initial assessment shows that Nano Covax is “relatively safe” for the volunteers, said Chu Van Men, Director of the Military Medical University’s Centre for Clinical Trials and Bioequivalence.
On March 11, the Hanoi-based Vietnam Military Medical University and the Pasteur Institute in Ho Chi Minh City have completed the administration of the first doses of Nano Covax in the second-stage human trials of this homegrown COVID-19 candidate vaccine, VOV News reported.
The inoculation for 560 volunteers, with 280 getting shots from each unit, was carried out in 12 days.
The 560 volunteers were divided into four groups, with 80 people injected with placebo and three other groups administered with 25mcg, 50mcg, and 75mcg doses. Among them, there are 105 people aged over 60, and the eldest is 76 years old. Some also have “not-too-serious” underlying health conditions like high blood pressure, blood lipid disorders, diabetes, and cardiovascular diseases.
Men said all the volunteers are currently in stable health condition, noting that their blood samples will be collected for antibody testing on the 28th, 34th, and 42nd days, as well as after three and six months, since the first jabs.
The third phase of human trials is expected to cover 10,000 – 15,000 people in both Vietnam and other countries.
The first-stage trials of Nano Covax showed that the vaccinated volunteers have had antibodies against the UK variant (B117). Phase 2 focuses on seeking antibodies against the UK and South Africa variants, according to Men.
Navo Covax, developed by the Nanogen Pharmaceutical Biotechnology JSC, is the first COVID-19 vaccine of Vietnam to be tested in clinical trials.
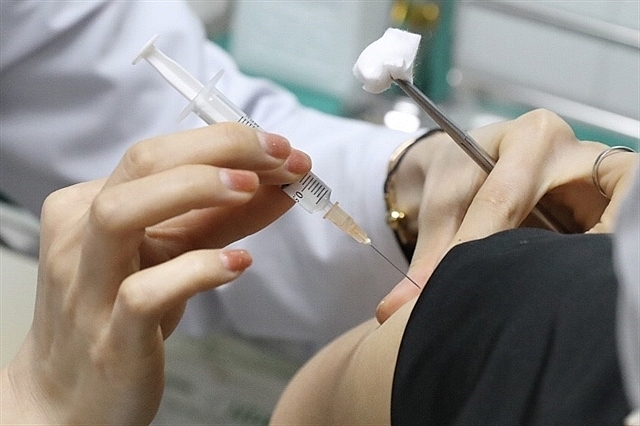 |
| A volunteer gets the second shot of Nano Covax COVID-19 vaccine on Thursday. — VNA/VNS Photo |
Previously, at a meeting of standing members of the National Steering Committee for COVID-19 Prevention and Control, a representative from the Ministry of Health said that the third phase of trials will take place from May to September, with the vaccine then registered for circulation in September, three months earlier than scheduled, Vietnam Plus reported.
Vietnam is one among 40 countries in the world that have started human trials of a COVID-19 vaccine, after successfully producing coronavirus test kits early into the pandemic.
The country also has several other COVID-19 candidate vaccines being developed, which are IVAC by the Institute of Vaccines and Medical Biologicals, VABIOTECH by the Company for Vaccine and Biological Production No 1, and POLYVAC by the Centre for Research and Production of Vaccines and Biologicals, Nhandan reported.
The hope for having the first batch of locally-produced Covid-19 vaccines at the end of the third quarter of 2021
The statement was made by Deputy Director of the Department of Science Technology and Training under the Health Ministry, Nguyen Ngo Quang, who is Chief of the Office of the National Programme on Vaccine Research and Development, at a meeting of the Standing Board of the Steering Committee for International Cooperation in Clinical Trials of Covid-19 Vaccines recently.
Quang said Vietnam is currently making research on three types of Covid-19 vaccine, The Star reported.
 |
| The second phase of human trial of home-grown Nano Covax is carried out at the Military Medical University on February 26. (Photo: VNA) |
Accordingly, the second phase of human trials on the NanoCovax vaccine, produced by the Nanogen Pharmaceutical Biotechnology, is being conducted, with the second dose to be injected on March 26.
The results of the second phase are expected to be announced in May as scheduled, while the third phase will be conducted from May to September. The vaccine is hoped to be registered for circulation in September, three months shorter than planned. Earlier, the trial time of the second phase was also shortened from six months to three months.
A system on monitoring and assessing vaccines’ protection efficiency in Vietnam and other countries is expected to be operated from September 2021 - September 2022.
Meanwhile, the first injections of the first phase of human trials on Vietnam’s second homegrown candidate vaccine COVIVAC, developed by the Institute of Vaccines and Medical Biologicals (IVAC), were made on March 15.
The first phase of human trials on the country’s third homegrown candidate vaccine, developed by VABIOTECH, is hoped to begin in April.
Standing members of the Steering Committee for International Cooperation in Clinical Trials of COVID-19 Vaccines agreed that the outcomes of pre-clinical trials of all three vaccines were assessed good thanks to Vietnamese units’ close coordination with prestigious vaccine producers and research units over the world and their compliance with international standards and procedures in vaccine research and development.
Speaking at the meeting, Deputy Prime Minister Vu Duc Dam, who is head of the National Steering Committee for Covid-19 Prevention and Control, hailed efforts made by the Ministries of Health and Science and Technology, and vaccine research and development units. He requested extra efforts to speed up domestically-developed vaccine research and production.
At the event, the Health Ministry affirmed that it has no policy that allows businesses and companies to import Covid-19 vaccines.
 |
| A healthworker gives the first shot in the first-stage safety study of Nanocovax - one of the four made-in-Viet Nam Covid-19 vaccines at the Military Medical Academy, Ha Noi, December 17, 2020. Photo: VNExpress.net |
Meanwhile, the first shipment of Covid-19 vaccines from the COVAX initiative to Vietnam originally slated for late March has now been delayed until mid-April due to production issues, a UNICEF official has confirmed.
Rana Flowers, UNICEF Representative to Vietnam, which is responsible for the procurement, transport, storage, and delivery of the vaccines, told Vietnam News on Wednesday that 811,200 Oxford/AstraZeneca vaccine doses – fewer than the original plan of 1.1 million doses for late March – are scheduled to arrive in the country in the next three weeks.
Around three million more doses will be arriving by the end of May, pending operational and supply constraints, a statement from UNICEF Vietnam reads.
Commenting on the adjustment of delivery date and initial quantity of COVAX vaccines to the country, Rana Flowers said the company has not been able to fulfil “the number they have predicted, and that they are going more slowly at this point in time.”
 | HCMC to start COVID-19 mass vaccination covering 8,000 people 8,000 doses of AstraZeneca vaccine are expected to be given to front-liners in Ho Chi Minh city, starting from March 22 to April 19. |
 | First batch of vaccine under Covax to arrives in Vietnam next month More than 811,000 doses of the AstraZeneca COVID-19 vaccine will be delivered to Hanoi next month via the WHO-led Vaccines Global Access (Covax) initiative. |
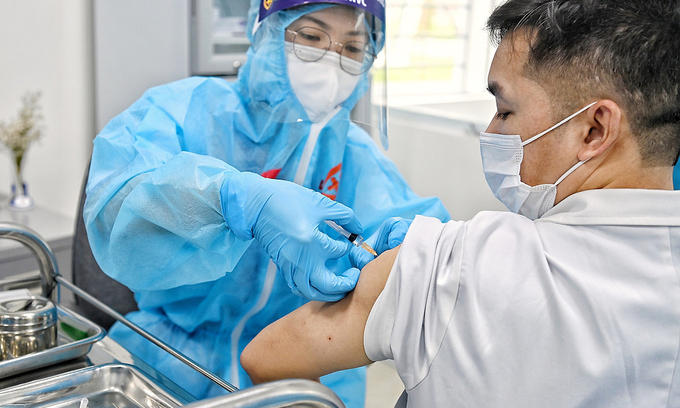 | Nearly 38.000 Vietnamese receive Covid-19 vaccine, more 3 provinces launch inoculation campaign this week As of March 23, more 1.829 people in Vietnam were administered the Covid-19 vaccine, raising the tally of recipients to 37.911. Three provinces of Quang ... |
Recommended
 Expats in Vietnam
Expats in Vietnam
Vietnamese Tet - Where “Friends from Afar” Find a Sense of Belonging
 Viet's Home
Viet's Home
Ho Chi Minh’s Legacy in the Land of Roses - Bulgaria
 Viet's Home
Viet's Home
Vietnam Continues to Work with UNESCO to Safeguard Cultural Diversity in the Digital Era
 Viet's Home
Viet's Home
Inviting Ancestors Home for Tet
 Viet's Home
Viet's Home
Universities In Vietnam Organize Tet Activities For International Students
 Viet's Home
Viet's Home
Zhi Shan Foundation Sent Tet gifts to Over 3,000 Children in Mountainous Region of Quang Tri
 Viet's Home
Viet's Home
Hai Phong Brings Lunar New Year Gifts to Disadvantaged Families in Tran Phu Commune
 Viet's Home
Viet's Home