India follows UK to grant emergency approval for AstraZeneca/Oxford Covid-19 vaccine
 |
| An Indian health-care worker and a patient were part of a test run of the country’s vaccine delivery network on Jan. 2 in New Delhi. Photo: RAJAT GUPTA/SHUTTERSTOCK |
Indian Information and Broadcasting Minister Prakash Javdekar said the AstraZeneca shot being produced locally by the Serum Institute of India Ltd. -- the world’s largest vaccine maker by volume -- was approved on January 1, according to Bloomberg.
“India is possibly the only country where four vaccine candidates are ready.” Javdekar said at the ruling Bharatiya Janata Party’s briefing on January 2 in New Delhi. “Yesterday one vaccine has been approved for emergency use, Serum’s Covishield.”
The Drugs Controller General of India has yet to formally announce the approval. Serum has an agreement with AstraZeneca to roll out at least one billion doses and has already made millions of shots. The move came just days after the U.K. regulator gave clearance to the vaccine, which is to roll out to Britain’s most vulnerable groups from January 4, Bloomberg reported.
The approval means India can begin to vaccinate its population of about 1.3 billion. That’s a daunting task given the country’s vast territory, limited infrastructure and patchy health networks.
AstraZeneca’s vaccine, which has the most supply deals globally, has been pegged as a more suitable shot for reaching people in the remote areas of India’s hinterlands than one developed by Pfizer Inc. and BioNTech SE that’s also being considered.
For the AstraZeneca/Oxford vaccine, the approval was “subject to multiple regulatory conditionalities”, the Indian government said, without giving details, according to Reuters.
India has reported more than 10.3 million COVID-19 cases and around 150,000 deaths, though its rate of infection has come down significantly from a mid-September peak.
The country hopes to inoculate 300 million of its 1.35 billion people in the first six to eight months of this year.
SII, the world’s biggest producer of vaccines, has already stockpiled about 50 million doses of the AstraZeneca/Oxford shot, which will be sold to the government at about 250 rupees ($3.42) per dose and 1,000 rupees on the private market.
Oxford-AstraZeneca Covid vaccine approved by UK regulator
 |
| An employee is seen at the Reference Center for Special Immunobiologicals (CRIE) of the Federal University of Sao Paulo (Unifesp) where the trials of the Oxford/AstraZeneca coronavirus vaccine are conducted, in Sao Paulo, Brazil, June 24, 2020. Photo: Reuters |
Oxford-AstraZeneca Covid vaccine was authorized on December 30 for emergency use in the U.K., marking another step in the global battle against the pandemic.
The shot is expected to be rolled out next week and will be added to a Covid-19 immunization program started by Britain in December with the Pfizer-BioNTech vaccine, CNBC said.
About 600,000 people in the U.K. have received the Pfizer inoculation, according to government statistics.
The Oxford-AstraZeneca vaccine is cheaper than others and does not need to be kept at ultra-low temperatures required by the Pfizer and Moderna vaccines.
In a statement, AstraZeneca said the first doses of the vaccine were being released on December 30 “so that vaccinations may begin early in the New Year.”
It added that it “aims to supply millions of doses in the first quarter” as part of its deal with the U.K. government to supply up to 100 million doses in total. As a two-dose vaccine, the agreement means up to 50 million people in the U.K., which has a population of around 66 million, could be inoculated.
However, the U.K. government said in a statement Wednesday that the Joint Committee on Vaccination and Immunisation, which advises it on immunization programs, had recommended that the “priority should be to give as many people in at-risk groups their first dose, rather than providing the required two doses in as short a time as possible.”
“Everyone will still receive their second dose and this will be within 12 weeks of their first. The second dose completes the course and is important for longer term protection,” it added.
Cold storage
 |
| Vials with a sticker reading, "Covid-19 / Coronavirus vaccine / Injection only" and a medical syringe are seen in front of a displayed AstraZeneca logo in this illustration. Photo: Reuters |
Pfizer’s vaccine requires subzero conditions for transportation and storage, while AstraZeneca’s can be stored at refrigerator temperatures and is also expected to be cheaper, Bloomberg said.
Yet clinical trial data indicates the Astra shot may be less effective than Pfizer’s and another similar vaccine from Moderna Inc., which each showed 95 percent efficacy in trials.
Initial data from Astra and Oxford in November raised concern over how much protection the vaccine would offer. The trials produced two different results from two dosing regimens. The partners said their vaccine was 90 percent effective when a half-dose was given before a full-dose booster, and that two full doses showed an efficacy of 62 percent.
While trial results published in The Lancet found the vaccine is safe and effective, more analysis will be needed to see how well it works in people over 55, among those at higher risk from the pandemic. A U.S. trial that aims to evaluate the shot in 40,000 people is ongoing and should clarify some of these questions, with results expected early in 2021.
Local doses
Human trials conducted by Serum in India have also been dogged by allegations from a volunteer who claimed serious side effects from the vaccine and is seeking compensation. Pune-based Serum has denied the claims and said the volunteer’s illness had nothing to do with the shot.
Serum has said half of any vaccine it produces will stay in India, with 100 million doses manufactured in December for the local inoculation drive, Chief Executive Officer Adar Poonawalla in an interview in November.
The Astra vaccine accounts for more than 40 percent of supplies going to low- and middle-income countries, based on agreements tracked by London-based research firm Airfinity Ltd./.
| AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines, primarily for the treatment of diseases in three therapy areas - Oncology, Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. |
 | Vladimir Putin to receive Russia’s Sputnik V vaccine The Russian president, Vladimir Putin, will receive the Sputnik V vaccine against coronavirus, the Kremlin spokesman told state TV channel Rossiya 1. |
 | COVID-19 Updates (Dec. 27): First three volunteers receive 50mcg dose of vaccine Three first volunteers were injected with the first shot of a dose of 50mcg of Vietnamese Nanocovax COVID-19 vaccine on December 26 morning. |
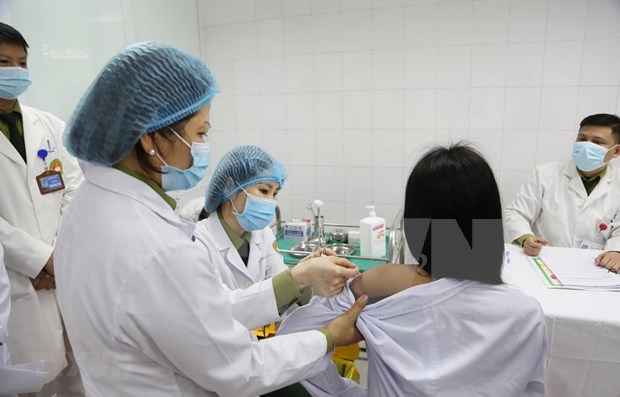 | Three volunteers injected 50mcg dose of Made-in-Vietnam Covid-19 vaccine On December 26 at the Hanoi-based Military Medical Academy, three local volunteers received a 50mcg dose of the Made-in-Vietnam Covid-19 vaccine Nano Covax. |
Recommended
 World
World
US Media Commend Vietnam’s Role in Global Peace Efforts
 World
World
Vietnam Officially Becomes Association Country of International Energy Agency (IEA)
 World
World
Key pacts signed as PM Modi hosts France's Macron for plane cooperation
 World
World
India, Canada commit to strengthening bilateral ties, discuss trade
 World
World
AI Summit India 2026 Live Updates: ‘Bringing the world together,’ PM Modi welcomes leaders as India hosts AI summit
 World
World
Safran ready to open India engine production in Rafale deal
 World
World
Nepal interim PM Sushila Karki thanks India for March support
 World
World