WHO approves China's Sinovac Covid-19 vaccine for emergency use
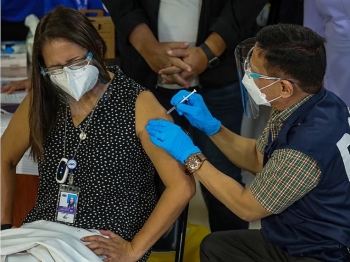
 |
| The CoronaVac vaccine against Covid-19, developed by China's Sinovac Biotech, won emergency use approval by the World Health Organization on June 1. Photo: EPA-EFE |
The UN health agency signed off on the two-dose vaccine, which is already being deployed in several countries around the world, the AFP reported on June 1.
"WHO today validated the Sinovac-CoronaVac Covid-19 vaccine for emergency use," it said in a statement.
The move gives countries, funders, procuring agencies and communities "assurance that it meets international standards for safety, efficacy and manufacturing".
Last month Sinopharm became the first Chinese vaccine to be approved by the WHO.
The organisation has also given emergency use listing to vaccines being made by Pfizer/BioNTech, Moderna, Johnson & Johnson, and the AstraZeneca jab being produced in India, the Republic of Korea (RoK) and the EU, which it counts separately.
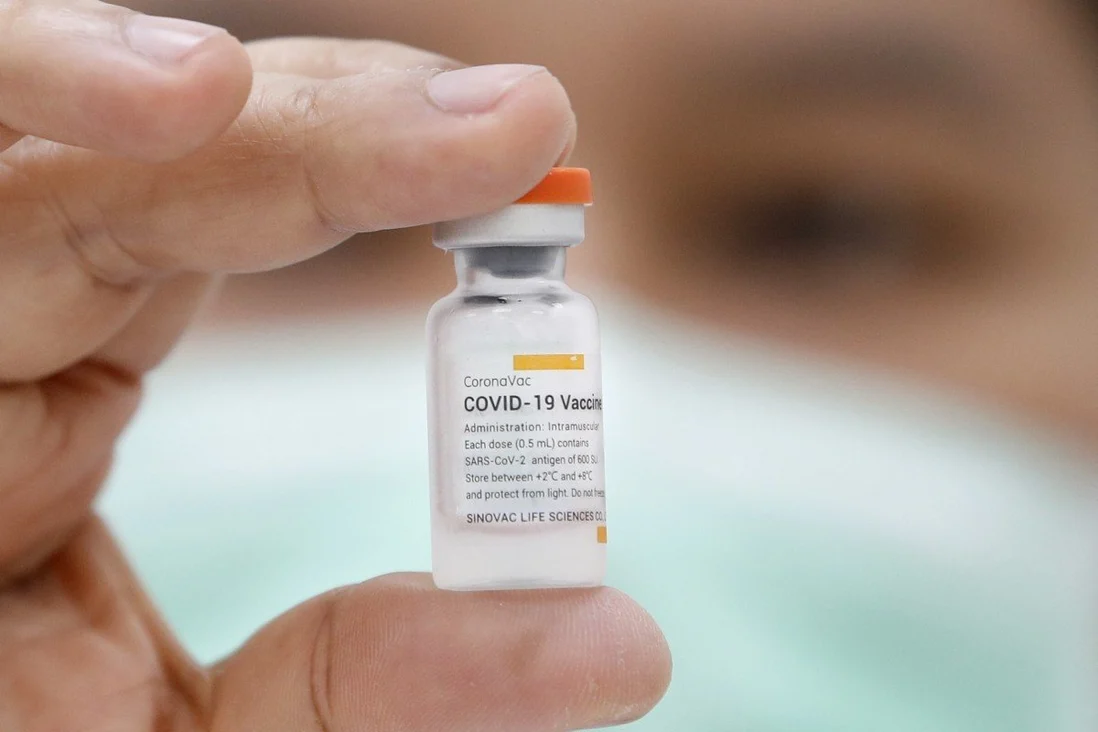
 |
| The Sinovac jab is already in use in 22 territories around the world. Photo: Reuters |
WHO's listing paves the way for countries worldwide to quickly approve and import a vaccine for distribution, especially those states without an international-standard regulator of their own.
It also opens the door for the jabs to enter the Covax global vaccine-sharing scheme, which aims to provide equitable access to doses around the world, particularly in poorer countries.
Currently only AstraZeneca and some Pfizer jabs are flowing through the scheme.
"The world desperately needs multiple Covid-19 vaccines to address the huge access inequity across the globe," said Mariangela Simao, the WHO's assistant director general for access to health products.
"We urge manufacturers to participate in the Covax facility, share their know-how and data and contribute to bringing the pandemic under control."
The Sinovac jab is already in use in 22 territories around the world, according to an AFP count.
Apart from China, the countries using Sinovac include Chile, Brazil, Indonesia, Mexico, Thailand and Turkey.
 |
| A woman receives China's Sinovac Covid-19 jab at the Central Vaccination Centre, inside the Bang Sue Grand Station, in Bangkok, Thailand, May 24, 2021. Photo: Reuters |
The WHO's Strategic Advisory Group of Experts on Immunisation have reviewed the jab and published their advice on its usage.
"WHO recommends the vaccine for use in adults 18 years and older, in a two-dose schedule with a spacing of two to four weeks," the agency said.
"Vaccine efficacy results showed that the vaccine prevented symptomatic disease in 51 percent of those vaccinated and prevented severe Covid-19 and hospitalisation in 100 percent of the studied population."
Reuters said the WHO's technical advisory group, which began meeting on May 5, made the decision after reviewing the latest clinical data on the Sinovac vaccine's safety and efficacy as well as the company's manufacturing practices.
WHO Director-General Tedros Adhanom Ghebreyesus welcomed the move, calling the vaccine safe and effective and noting its easy storage requirements make it suitable for low-income countries.
"It's now crucial to get these lifesaving tools to the people that need them quickly," he told a briefing./.
 | Indonesian nurse dies 9 days after receiving Chinese vaccine An Indonesian nurse has died nine days after being injected with the Chinese vaccine made by Sinovac Biotech Ltd. |
 | No new coronavirus cases reported for the first time in China in 2 months On Monday (February 8), official data shows that there is no new locally transmitted mainland coronavirus cases in China, adding a positive possibility that the ... |
 | Pfizer-BioNTech’s COVID-19 vaccine meets prescribed success criteria in the clinical study, paving the way for the agency to green-light distribution as early as this weekend. ... |
Recommended
 World
World
US Media Commend Vietnam’s Role in Global Peace Efforts
 World
World
Vietnam Officially Becomes Association Country of International Energy Agency (IEA)
 World
World
Key pacts signed as PM Modi hosts France's Macron for plane cooperation
 World
World
India, Canada commit to strengthening bilateral ties, discuss trade
 World
World
AI Summit India 2026 Live Updates: ‘Bringing the world together,’ PM Modi welcomes leaders as India hosts AI summit
 World
World
Safran ready to open India engine production in Rafale deal
 World
World
Nepal interim PM Sushila Karki thanks India for March support
 World
World